PARTICIPANT IN PHASE 3 COVID-19 VACCINE TRIAL
Human trials are mandated before a vaccine receives approval for general use. There are almost a dozen coronavirus vaccines in late-stage trials. Along with a friend, I volunteered for a Phase 3 COVID-19 Vaccine Trial and just received the first of two injections.
Why? Two reasons include contributing to the advancement of drug approval and having a chance to receive a vaccine months before it will be available to the general public.
These trials are double-blind studies, meaning some of the volunteers receive a placebo. The staff and recipients do now know which.
Our regional test site was running trials for both the single-injection Johnson & Johnson vaccine and the Oxford-Astra Zeneca two-injection vaccine. I chose to participate in the later. Why? The Johnson & Johnson study pool is 50:50 vaccine/placebo while the Oxford-Astra Zeneca study is a 2 out of 3 pool, meaning 67% of the volunteers receive the trial vaccine.
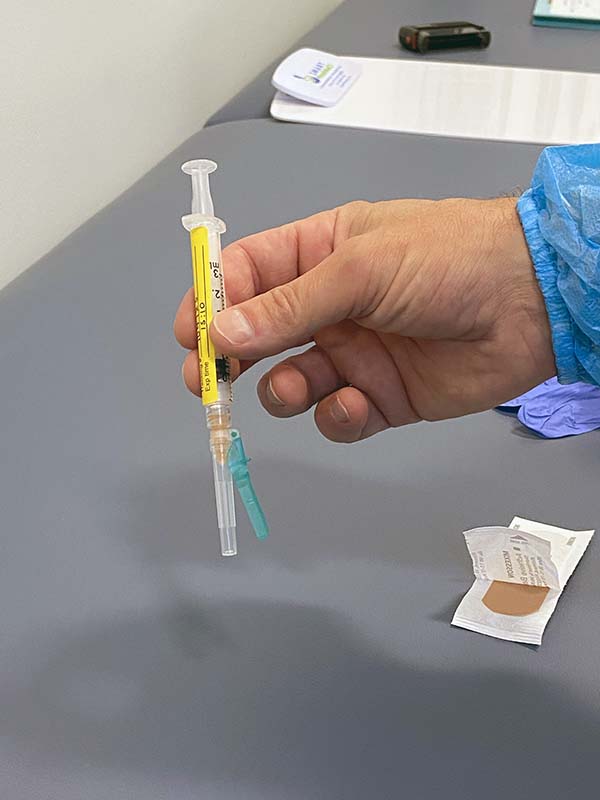
Here is the syringe that I received. Vaccine or Placebo?
What are the odds that my friend and I both received the trial vaccine, or at least one of us did? Conversely, what are our odds that we both received the placebo?
Given that a software random number generator generates the sample codes, we can assume a near-perfect 67% chance of any individual receiving the trial vaccine and a 33% chance of receiving the placebo. This Yes/No scenario makes it a binomial.
Since my friend and I are two samples with equal probability, multiply 0.67 * 0.67 to get 0.45 or just under a fifty percent chance of both of us received the trial vaccine. Performing the same calculation for the placebo, we calculate 0.33 * 0.33 to get 0.11 or just over a ten percent chance that we both received the placebo.
To calculate the odds that at least one of us received the active vaccine, we consult a calculator. Using the online Binomial Probability Calculator by Stattrek.com, I entered 0.67 as the probability of a positive test, 2 for the number of trials, and 1 for the number of successes.
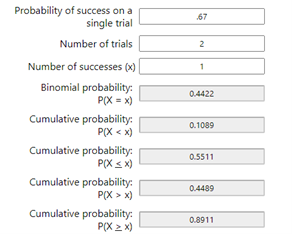
The probability that P(X >= 1) is 0.89 or almost ninety percent chance that at least one of us received the trial vaccine.
I have prepared a table comparing the probabilities for the two of us in the Astra Zeneca 2 out of 3 trial and for the competing 50:50 Johnson and Johnson trial:
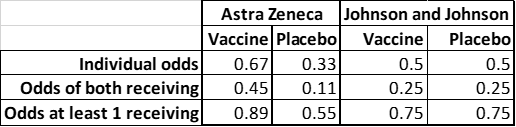
We will be informed if we received the actual vaccine after the study concludes months from now. For now, at least we know that odds.